
The Drug Enforcement Administration (DEA) has released a final rule that addresses forthcoming regulations regarding federally legal cannabis research.
The announcement of the final rule follows the United Nations Commission for Narcotic Drugs’ vote to remove cannabis from its list of most dangerous drugs, and the U.S. House and Senate’s passage of cannabis research bills.
RELATED: U.N. Vote on Cannabis: Is This the Beginning of the End of the Controlled Substances Act?
In the public comment section of the rule, which goes into effect Jan. 19, 2021, DEA explains that “past experience in the manufacture of controlled substances” will be a factor the agency will consider when reviewing applications. The rule, citing the U.S. Congress, states:
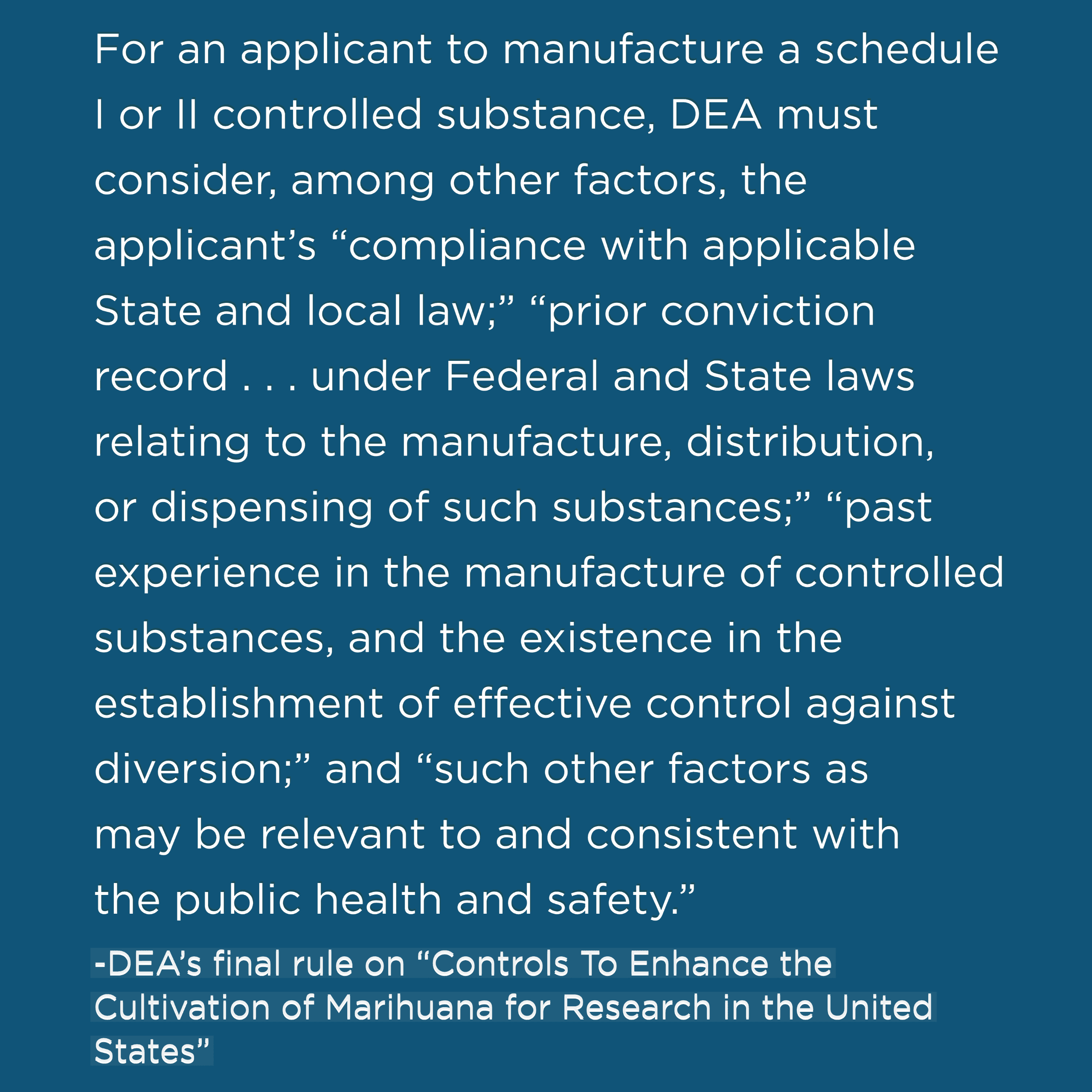
The rule continues: “…An applicant that has manufactured marijuana without obtaining a DEA registration has violated Federal law, … regardless of whether that manufacturer has violated the laws of the State in which the applicant is located. … While the DEA Administrator has discretion to weigh the statutory factors and any one factor need not be dispositive, an applicant’s prior compliance with Federal law is a relevant consideration when determining whether to grant an application for registration.”
Some see DEA’s registration criteria as a red flag. Henry Baskerville, a partner at Fortis Law Partners provided the following statement to Cannabis Business Times and Cannabis Dispensary.
“The federal cannabis cultivation license allows companies to grow cannabis for use in clinical research. Excluding those who currently have state cultivation licenses is excluding people and companies that have the experience effectively cultivating cannabis. This makes no sense from a clinical perspective and is simply punitive action by the DEA against persons and businesses who have been participating in legal activity under state law.”
Tyler Williams, founder and chief technical officer at Cannabis Safety & Quality, said DEA’s final rule may be in part an act of self-preservation.
“I think that with the MORE [Marijuana Opportunity Reinvestment and Expungement] Act gaining traction and federal legalization around the corner, the DEA is kind of stuck in a corner,” Williams told CBT and CD. “… And when you back an agency in a corner after you’ve taken out a majority of the product that they control, which is cannabis—once you legalize it federally, they no longer have control over it.
“That means a large portion of their budget goes away in theory, or it should. So, you have this agency that is kind of clawing their way back and trying to gain control, so I think, in my personal opinion, that this is a way for them to say, ‘Ok, well, if we go the route of descheduling cannabis, we can do it our way and still have control over it.’ And that’s exactly what it sounds like they’re trying to do.”
However, some companies are moving forward in the application process. In 2019, DEA selected MedPharm Research as an applicant to obtain a research license. MedPharm Holdings CEO Albert Gutierrez said in an email to CBT and CD that MedPharm Research operates separately from MedPharm’s commercial manufacturing operations, which produce Aliviar, Batch and Become products. As MedPharm Research does not produce commercial cannabis products, Gutierrez said it follows all federal laws.
“There are always obstacles in new industries and areas of opportunity, but we remain confident in our capabilities, know-how, and relationships with various universities to carry out a great program that will help shed light on the medicinal value of cannabis,” Gutierrez said. “We welcome the opportunity to collaborate with the DEA and others on this venture and look forward to what is to come.”
Regarding the final rule language addressing criminal convictions, industry stakeholders have shared, in reference to the Marijuana Opportunity Reinvestment and Expungement (MORE) Act, that when federal officials make past criminal convictions a factor in whether to allow industry participation, it reduces social equity.
Dasheeda Dawson, cannabis program supervisor for the city of Portland, Ore., told CBT and CD contributor Raj Chander via email: “Over the past few years, the definition of cannabis equity has centered on providing opportunities to those most adversely harmed. Undoubtedly this starts with those who were previously arrested or incarcerated for cannabis.”
The public comment section of the final rule also addressed cannabis product quality. “Some commenters stated that the current quality of marihuana produced for Federal research is of poor quality,” DEA stated, referring to cannabis produced at the University of Mississippi.
In response, the agency wrote: “The purpose of this rule is to increase the number and variety of marihuana growers in order to diversify the supply available to researchers. As proposed in the NPRM and finalized in this rule, one of the selection criteria for marijuana grower applicants is the “applicant’s ability to consistently produce and supply cannabis of a high quality and defined chemical composition.”
The agency also noted that someone commented that DEA should extend “the time period for providing notice of a harvest to five days beforehand, instead of 15 days beforehand,” as DEA had previously indicated was its plan. The agency’s response stated that “DEA has concluded that a five-day notice period will not provide sufficient time to make the arrangements needed to travel to a grower and attend a harvest.”
The final rule is published in the Federal Register.
