The explosive, unregulated growth of delta-8 tetrahydrocannabinol (THC) is coming with a dangerous consequence: Poison control centers across the country are reporting a rise in calls from those who have ingested the cannabinoid.
At least four states have issued public warnings regarding the cannabinoid, according to local news reports: Michigan, West Virginia, Virginia and North Carolina.
So far this year, as of July, the North Carolina Poison Control Center reported 157 cases related to delta-8, according to the Winston-Salem Journal. Virginia is reporting “dozens” of calls this year.
The rise in calls can be attributed to a number of factors, from the market’s lack of standards to delta-8’s accessibility to minors.
But perhaps the most concerning cause for an increase in delta-8 poison center calls is due to mislabeling–in some cases, consumers hoping to buy these products may be purchasing unregulated, illegal cannabis without knowing.
“This is part and parcel for what happens when you have an unregulated market,” says Jonathan Miller, general counsel for U.S. Hemp Roundtable.
Mislabeling Plays a Role
A recently published report shines a light on just how prominent mislabeling is in the industry. Leafreport.com, a peer-reviewed watchdog website for the cannabidiol (CBD) industry, found that more than half of the 38 products it tested had illegal levels of delta-9 THC. In addition, only 32% had the advertised amount of delta-8. The rest were off by 10.7% to 102.7% from the label.
And Wayne State University, based in Michigan, reports that law enforcement agencies in Michigan have seized delta-8 products that were falsely labeled as CBD products.
Lev Spivak-Birndorf, Ph.D., chief science officer at PSI Labs, a Michigan-based cannabis testing lab, says the mislabeling of these products could be linked to the risks associated with producing delta-8, as byproducts can be left during the process of converting it from CBD or delta-9 THC.
For example, when labs extract cannabis to make other oils, it’s a more straightforward process, as there are already established and known solvents and methods, like butane or ethanol extraction, that labs can use to remove byproducts, Spivak-Birndorf says.
“But once you get into the process of converting CBD into delta-8, it’s called chemical synthesis,” he says. “You’re essentially taking one molecule and performing a chemical reaction that’s causing it to change into another molecule via that reaction.”
There are several standard operating procedures (SOPs) that can be used to extract delta-8; however, there are many “variables in terms of what can be left over in the product and how dangerous the substance is that labs use to catalyze that reaction and make it occur at a speed that’s acceptable for production,” he says.
PSI Labs has not yet conducted extensive research into what type of contaminants can be found in delta-8 products due to costs; however, the laboratory has brought in off-the-shelf delta-8 products from places like vape shops and gas stations for testing, and they’ve discovered discrepancies in the products.
“We often find that some of [the products] are very pure delta-8, but still contain traces of delta-9 that are technically above the legal 0.3% THC limit,” he says. “It’s just a mixed bag, and that’s what I think is so risky about it, is that it’s very uncontrolled in terms of these processes, where one product could be pretty genuine to its label, but another one might be very different.”
RELATED: Understanding Delta-8-THC: Where Does It Come From?
Allison Justice, Ph.D., CEO of The Hemp Mine, previously told Hemp Grower that in the delta-8 extraction process, the final distillate ends up being 60% to 70% delta-8 and roughly 2% to 6% delta-9. Suppose a company wants to make a compliant product with the U.S. Department of Agriculture’s (USDA’s) final rule on hemp. In that case, they will then run the distillate through extensive chromatography to remove the delta-9 or dilute it down to ensure it’s under the 0.3% THC limit, she said.
But Spivak-Birndorf says PSI Labs has found off-the-shelf delta-8 products that contain 20% delta-9, as well as a significant amount of CBD, meaning that whatever reaction pathway they chose, they didn’t finish it and left it “half-cooked,” he says.
“This raises lots of questions about what residual other byproducts might be in [the delta-8 products] that are sort of not familiar cannabinoids, but maybe harmful substances, like heavy metals, which can often be used in these processes,” he says.
Spivak-Birndorf says PSI Labs has come across many delta-8 vapes over the 0.3% THC limit; however, he thinks the real risk with mislabeling is with edibles.
“Once you eat [the edible], that’s how much you eat, and you really can’t go back from there. So, that’s where testing is absolutely critical,” he says. “You really need to test things in a fashionable manner to make sure that there’s nothing wrong with your process. Other packaged and ready-made goods go through many good manufacturing practices (GMPs) and Food and Drug Administration (FDA) guidelines before they get to the shelf. In the cannabis industry, a lot of that stuff is non-existent or just catching up.”
Spivak-Birndorf says there is a need for third-party, end-product testing on a batch basis to ensure the products are correctly dosed before hitting shelves because there are not many regulated delta-8 testing and measurement practices in place.
“All of this is an important part of a good manufacturing practice of what goes into all the packaged goods we consume,” he says. “And so really, it should be implemented for delta-8 or delta-9, just like anything else.”
“There’s no reason to limit ourselves in terms of the cannabinoid toolbox,” he adds. “Whether it’s naturally occurring, semi-synthetic or even synthetic, it’s very important to know exactly what we’re dealing with and what quantities we’re dealing with if we want to avoid unknown adverse reactions and keep people as safe and healthy as possible.”
Minors the Main Victims
According to the Wayne State University report, in a nearby state, there were two reported cases of severe adverse reactions to delta-8 in children who ingested their father’s edibles purchased at a vape shop.
The report says that because delta-8 products are packaged in similar forms of CBD, teens and young children have a higher risk of using the compound accidentally. “THC use in children can cause low blood pressure, difficulty breathing, severe sedation, coma, and psychological effects. Long-term effects in children are not known,” the report says.
According to the National Capital Poison Center, because delta-8 products are largely unregulated, “the packaging may not be child-resistant and may contain cartoon-like images or other features that may be appealing to children.”
On its website, the National Capital Poison Center offers this anecdote: “A 3-year-old boy was brought to an Emergency Department after eating an unknown amount of delta-8 THC gummies belonging to his mother. The gummies were packaged in a container with a twist-off top, and the boy was able to open the container by himself. The child experienced vomiting but was eventually released from the hospital. His mother was cited for child abuse and neglect.”
Market Regulation Needed
Spivak-Birndorf says there is an increased need for regulation, as it is difficult to trace where these unsafe delta-8 products are coming from.
“While some of these companies have an online presence and show current testing and are actually responsible, for every one of those, there’s probably, and I would guess, numerous [companies] that are flying by night and just not checking their work,” he says. “Or they’re not concerned about quality control checks for their product, which they should have. So, definitely without that regulatory push to do all that stuff, it’s unrealistic to think that [everyone] is just going to take that upon themselves.”
As previously reported by Hemp Grower, Michigan has recently joined numerous other states in regulating delta-8, as Gov. Gretchen Whitmer signed legislation into law July 13, prohibiting businesses from selling delta-8 products without proper licensing from the Michigan Marijuana Regulatory Agency, and the products must be tracked and tested like any other cannabis product.
Additionally, “the new law also bars businesses without state cannabis licenses from producing and selling any other potentially intoxicating cannabis compounds,” Hemp Grower previously reported.
And in late April, Hemp Grower reported that Washington’s Liquor and Cannabis Board (LCB) issued a policy statement, stating that “Delta-8 THC, as well as derivatives, extracts, cannabinoids, isomers, and CBD isolate from hemp or other sources that are genetically or chemically altered into compounds may not be produced or processed in LCB licensed facilities, and may not be sold in licensed marijuana retail stores.”
However, this does not necessarily mean delta-8 is outright banned in the state, as the LCB’s notice “was only an interpretation of the law as it relates to the legal cannabis market,” Hemp Grower reported.
Washington and Michigan are just two of nearly 20 states that have already regulated the cannabinoid. According to Hemp Grower, 15 states have issued bans on delta-8, while six additional states have pending legislation to regulate the cannabinoid and related THC isomers.
Miller says the U.S. Hemp Roundtable has been working with states on developing laws that regulate delta-8 under the cannabis market because of its psychoactive properties. So far, Michigan, Oregon and New York have passed similar laws.
“I would very much hesitate buying a delta-8 product” in the current market, Miller says. “I would only buy things available in a dispensary that have been proven to undergo the same type of testing [as regulated cannabis].”
Not Just Delta-8
Delta-8 isn’t the only cannabinoid responsible for an uptick in poison center calls this year.
As of June 30, the American Association of Poison Control Centers (AAPCC) reported that poison control centers across the country had managed 2,158 cases in 2021 related to CBD. This is nearly on par with the 2,226 calls placed the entire year in 2020.
For perspective, in June 2020, poison control centers handled 181 cases related to CBD; in June 2021, that number jumped to 423.
The AAPCC offers this explanation on its website for the increase in calls: “…[S]ome products contain more CBD than what is on the label, more THC than labeled, or other chemical compounds/drug ingredients that are not listed at all. Consumers have no way of knowing whether the product is contaminated with other chemicals and drugs or labeled correctly.”
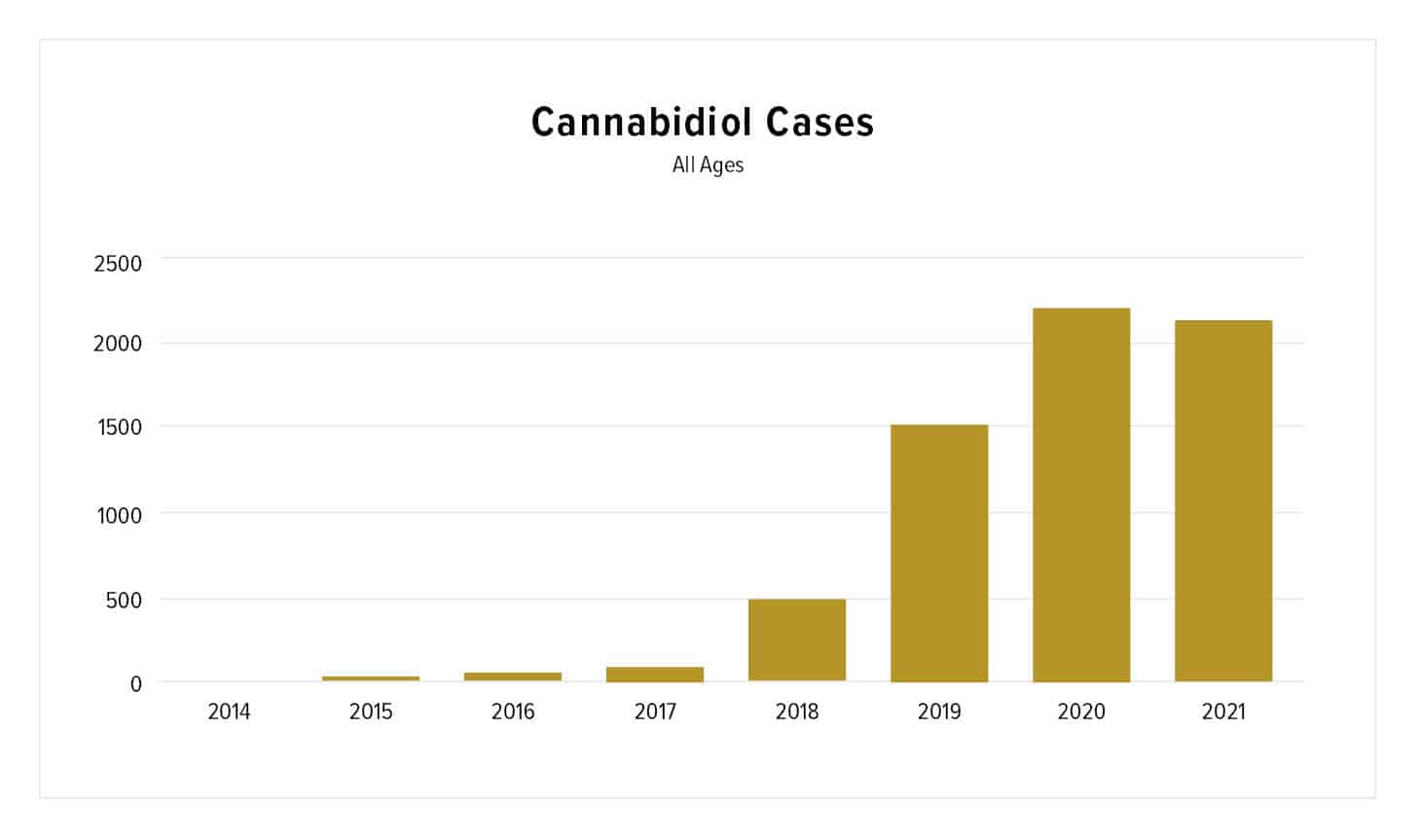
© Chart Courtesy of The American Association of Poison Control Centers
Miller points out that CBD is “a safe compound, and study after study have demonstrated its safety.” But without clear regulations or even guidance on how to produce CBD products, it’s possible the products contributing to poison control center cases don’t comply with GMP safety standards, Miller says.
“The challenge is that we’re in an unregulated marketplace,” he says. “These kinds of problems are hopefully going to spur Congressional action that will pass bills to develop a regulatory structure.”
Miller adds that the Hemp Access and Consumer Safety Act moving through the U.S. Senate would require the U.S. Food and Drug Administration to develop a regulatory pathway for CBD to be used in food, beverages and dietary supplements.
You can reach your local poison control center by calling the Poison Help hotline: 1-800-222-1222. To save the number in your mobile phone, text POISON to 797979.
